Physics of Capnography
Water absorbs IR light but minimum at 4.3µ m
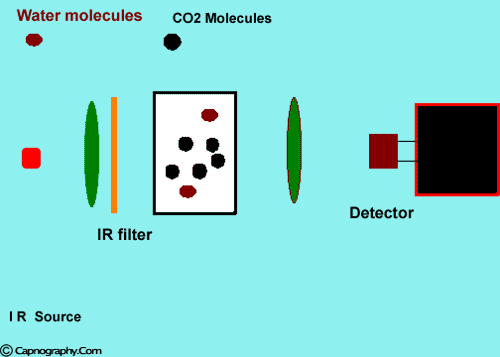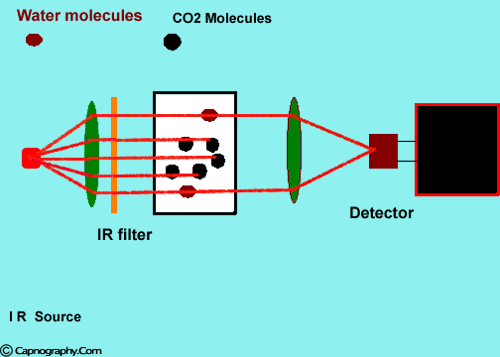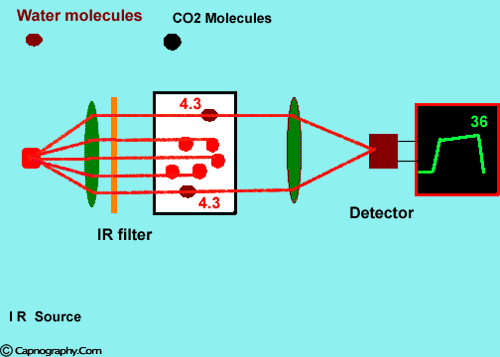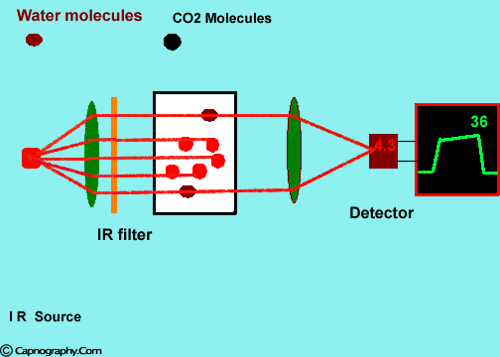
How does water vapor affect CO2 readings? |
|
Water can condense on IR cell and interfere with CO2 measurements |
|
Decrease in partial pressure of water due to temperature differences between the patient’s airway and the measuring unit can increase CO2 values. |
|
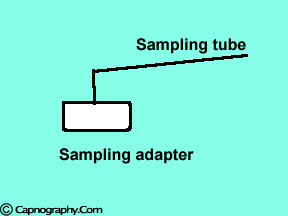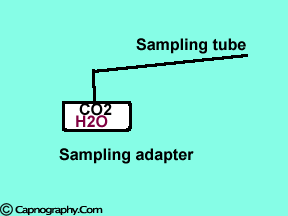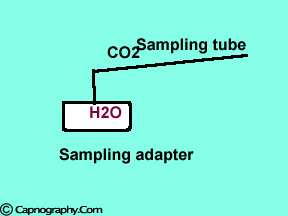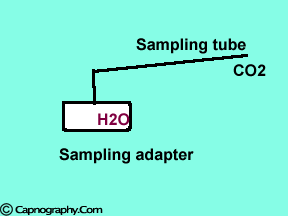
Water can condense and clog the sampling line. |
Changes in water vapor alter CO2 readings
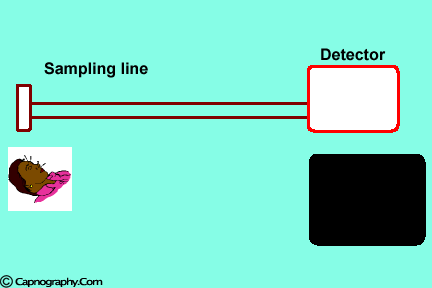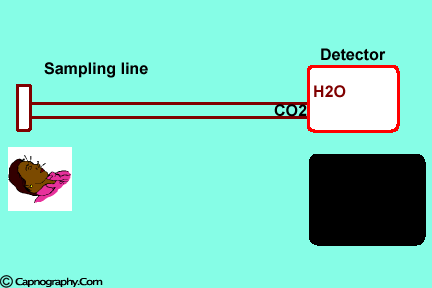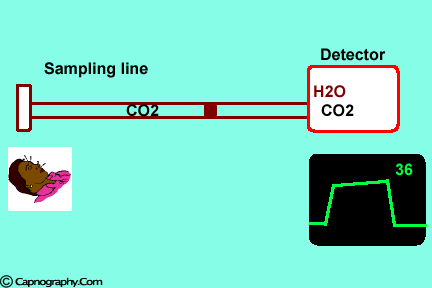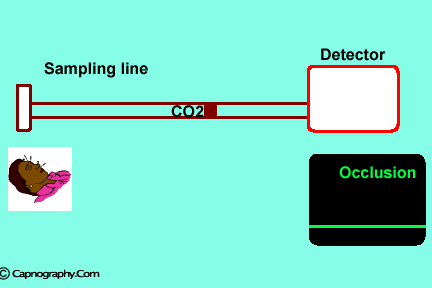
| Position the sampling vertically upwards2 | Use water filters at both ends of sampling tube |
|
 |
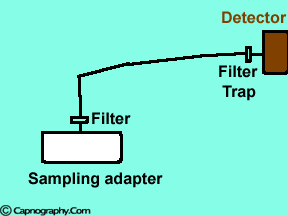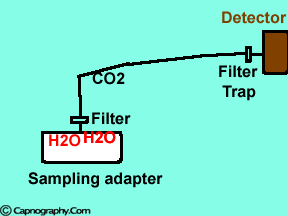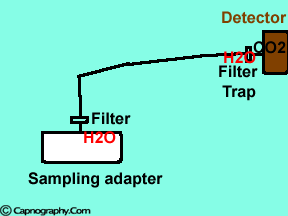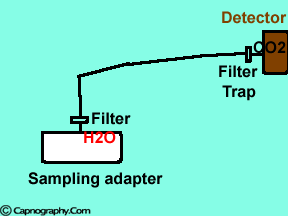 |
Oridion, Inc has incorporated a Filterline airway adaptor (Microstream airway adapter) coupled with low flow Microstream technology to minimize the water vapor problem.1 The airway adapter has three channels positioned in the center lumen of the adapter, facing different directions. This eliminates the problem associated with conventional sidestream airway adapters, whose upright orientation was mandatory for proper functioning, where any orientation other than upright may result in occlusions. Channel openings are made of small diameter hydrophobic material. These openings are specifically designed so that even when all but one channel is occluded, the system will continue to draw the sample breath, without secretion penetration into the sampling circuit. This allows for minimization of liquid and secretion blockage or penetration into the sampling circuit, thereby extending the life of the circuit. Most importantly, the multiple sample ports also permit the airway adapter to be inserted in any and all orientations.1

Water vapor can affect CO2 readings in two ways.
1. Effect of condensed water:
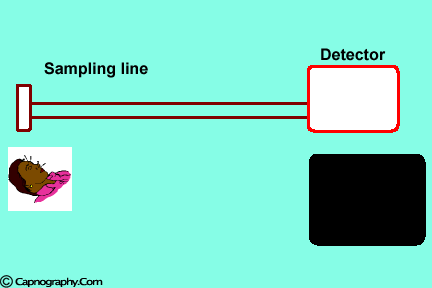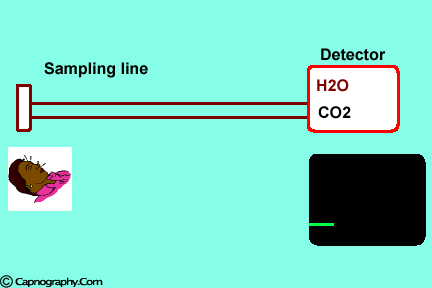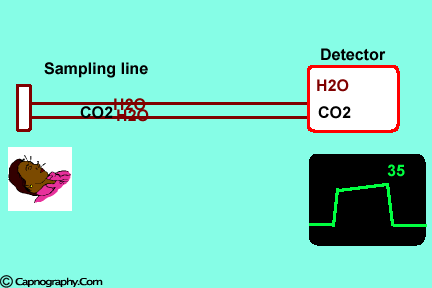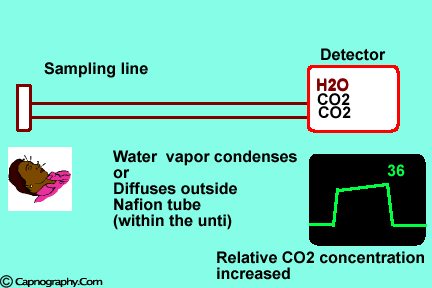
Water vapor may condense on the window of the sensor cell and absorb IR light, thereby produce falsely high C02 readings. This effect can be prevented by heating the sensor above body temperature (main-stream sensor units) or by removing the excess water vapor before it reaches the sensor (side-stream sensor units). Thus, some side stream-sensor units use a special sampling tube made of Nafion, a semipermeable polymer that selectively allows water vapor to pass from the interior of the tube to the exterior. Other side-stream units interpose liquid traps or moisture-absorbent filters between the sampling tube and the analyzer that help to remove excessive water and secretions, thus the optical system is protected.2-4
2. Effect of water vapor.
Main-stream IR analyzers measure the gas in the breathing circuit, which is generally saturated at body temperature. The exact water vapour pressure in the breathing circuit will depend on many factors including the use of heated humidification, fresh gas flow, length of time in use and temperature.3 In side-stream sensors the temperature of the sampling gases may decrease during the passage from the patient to the unit, resulting in a decrease in the partial pressure of water vapor. This can cause an apparent increase in C02 concentration of about 1.5-2%.4,5 Further, if Nafion tubing is used in the sample catheter, then it actually equilibrates the water vapor pressure inside the tubing to that of outside the tubing.3 Therefore, PETCO2 measurements should be corrected for the effects of water vapor, in accordance with the type of analyzer used, and the manufacturer’s instructions.4,5
References:
1. Coleman Y, Krauss B. Microstream capnography technology: A new approach to an old problem. J Clin Monit 1999;15:403-9.
2. Carbon dioxide monitors. Health Devices 1986;15:255-85.
3. Raemer DB, Calalang I. Accuracy of end-tidal carbon dioxide tension analyzers. J Clin Monit 1991;7:195-208.
4. Paloheimo M, Valli M, Ahjopolo H. A guide to CO2 monitoring. Finland: Datex Instrumentarium, 1988.
5. Fletcher R, Werner O, Nordstrom L, Jonson B. Sources of error and their correction in the measurement of carbon dioxide elimination using the Siemens-Elema CO2 analyzer. Br J Anaesth 1983;55:177-85.
6. Bhavani Shankar K. A method to prevent occlusion of CO2 sampling tubes. Can J Anaesth 1997:44.

 Twitter
Twitter Youtube
Youtube










Comment on “Effect of water vapor”