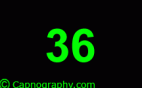Clinical Aspects
Bhavani Shankar Kodali MD
Capnography provides three sources of information
| From Numbers- PETCO2 values- Capnometry | |
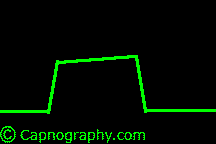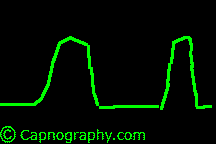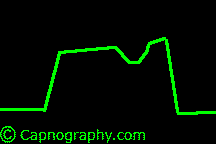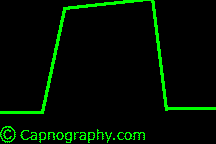
| From Lines and Curves–Shapes of capnograms – Capnography | |
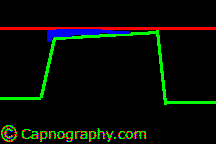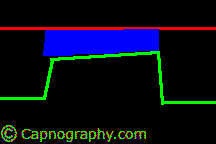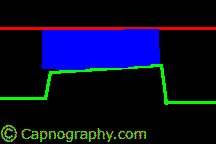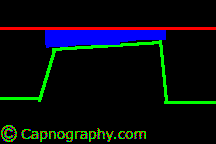
| From (a-ET)PCO2 gradients or differences- Alveolar dead space | |
|
|
|
|
|
|
|
|
|

 Twitter
Twitter Youtube
Youtube